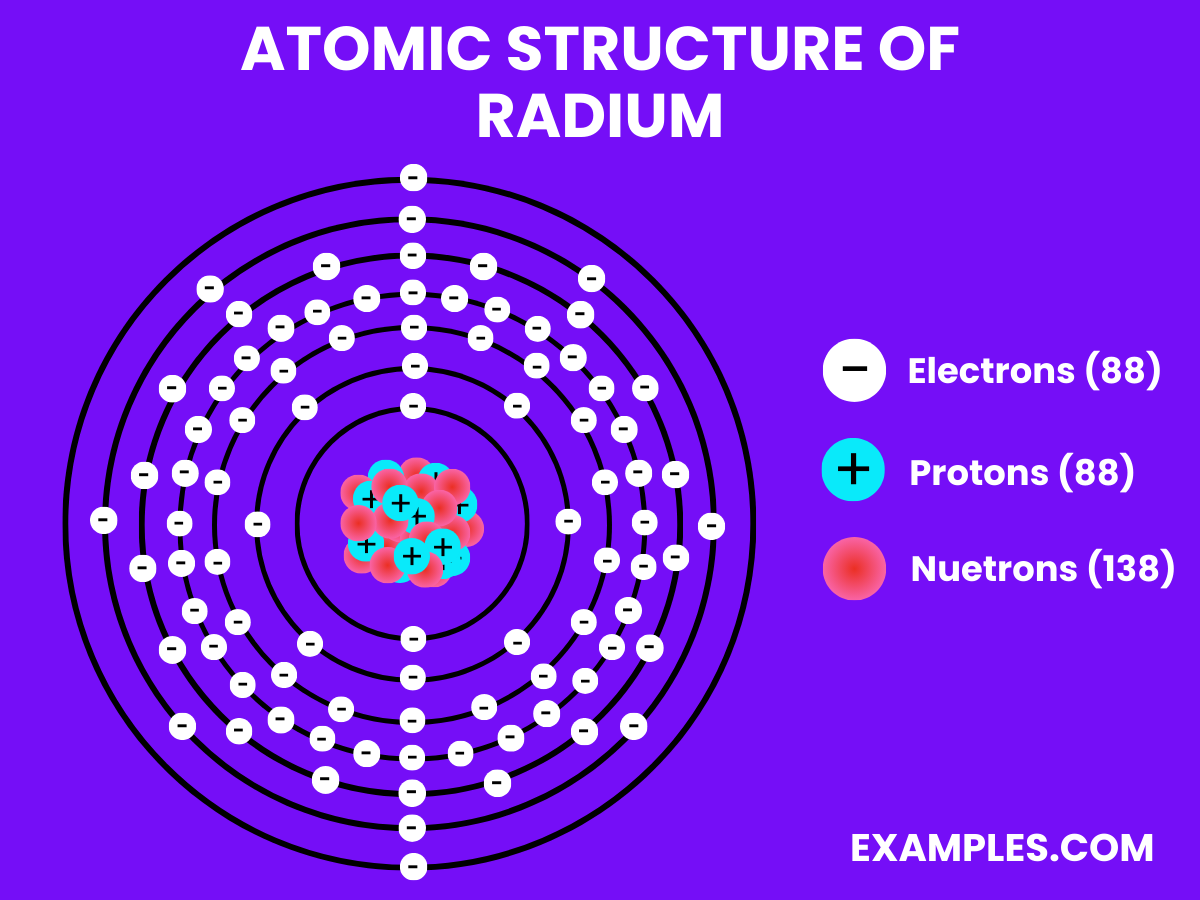
Radium oxide is an inorganic compound of radium and oxygen with the chemical formula RaO.
Synthesis
The compound can be obtained by heating metallic radium in air:
- 2Ra O2 → 2RaO
This reaction also produces radium nitride and possibly radium peroxide:
- 3Ra N2 → Ra3N2
- Ra O2 → RaO2
Chemical properties
Radium oxide can react with water to form radium hydroxide:
- RaO H2O → Ra(OH)2
Uses
It is often used as a precursor to create other radium compounds that are used in radiation therapy.
References